 Remember that we do not “feed” plants. The major thing that separates plants from animals is that they feed themselves through a process called photosynthesis. This is where they capture the energy from the sun (or other light source) and, in the chlorophyll (green colored) molecule in leaves, they pull together a variety of chemical elements and water to trap that energy. The resulting sugars and carbohydrates are then the basis of almost all living things on earth.
Remember that we do not “feed” plants. The major thing that separates plants from animals is that they feed themselves through a process called photosynthesis. This is where they capture the energy from the sun (or other light source) and, in the chlorophyll (green colored) molecule in leaves, they pull together a variety of chemical elements and water to trap that energy. The resulting sugars and carbohydrates are then the basis of almost all living things on earth.
Animals, including we humans, feed on this plant material and convert that energy into proteins and other tissues. We can gain transfer the energy of the sun into our bodies by eating either plant or animal materials.
As an animal, if I eat pounds and pounds of sugar filled donuts or steaks, I will grow bigger. On the other hand, if I apply more and more fertilizer onto my plants, they may or may not grow. They can only use these nutrients if the levels of light, chlorophyll, water and temperature are at the required minimum for that particular plant species.
If I put too much of some types of fertilizers on my plants, they may even die. This happens because many of the elements needed by plants fall into the “salts” category of chemicals. If a clump of granular fertilizer or a wad of fresh animal manure comes into the contact with plant roots, it may cause the plant to “burn”. What is really happening, of course, is that the salts in the fertilizer or manure will pull the water out of the roots and cause them to die. As a result, the leaves and stems that were relying on those dead roots for water must also die.
Before starting a garden, lawn, flower bed or other planting, it is a good idea to take a soil test. The results will show how much phosphorus, potassium, calcium, and magnesium is present in the soil. It will also give you the pH, the type of soil and a specific fertilizer recommendation for the proposed crop i.e. flowers, fruit, vegetables, lawn, etc.
On a standard soil test, however, nitrogen levels will NOT be tested. This is because nitrogen is very water soluble and moves through the soil quickly. The amount present in your soil may vary greatly over short periods. So, by the time the soil test result gets to you, the level of N may have changed considerably. The sample results will make a nitrogen recommendation based on the amount commonly needed by the crop to be grown. For examples, a typical Kentucky bluegrass lawn will generally need 3 to 6 pounds of nitrogen per 1,000 square feet per summer depending on whether it is irrigated or not.
The other major nutrients such as phosphorus or potassium are held more tightly by the soil particles and remain more constant in the soil over the growing season. Many soil tests, especially in areas with clay-type soils, will report high levels of potassium or phosphorus in gardens or beds that have been routinely fertilized. In such cases, the fertilizer recommendations may show that no more needs to be added for that growing season.
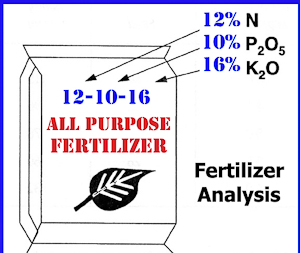 If you go to your local garden center, you will find that most fertilizers have three numbers on the label. An example would be a 12-12-12 fertilizer which would contain 12% each of nitrogen (N), phosphorus (P) and potassium (K). In a 100 pound bag, we get 12 pounds of each of these three nutrients. (Technically, it is a little more complicated than that but the home gardener does not have to worry about it since the recommendations take the chemistry into account for you.)
If you go to your local garden center, you will find that most fertilizers have three numbers on the label. An example would be a 12-12-12 fertilizer which would contain 12% each of nitrogen (N), phosphorus (P) and potassium (K). In a 100 pound bag, we get 12 pounds of each of these three nutrients. (Technically, it is a little more complicated than that but the home gardener does not have to worry about it since the recommendations take the chemistry into account for you.)
If your soil test says that you only need to add nitrogen or just nitrogen and phosphorus or nitrogen and potassium, there are special fertilizers available to fill these needs. Generally, these are called single nutrient fertilizers. They may be purchased separately and used in combination to meet your specific needs.
When only nitrogen, for example, is needed, there are several alternatives. Ammonium sulfate has an analysis of 21-0-0 (21% N) and is preferred by plants such as rhododendrons, azaleas and blueberries that need an acid soil. Urea (45% N) and urea formaldehyde (38% N) are synthetic organic compounds that give a slightly acidifying effect. Sodium nitrate (16% N) and calcium nitrate (15% N) cause the soil to become slightly less alkaline. You can tailor your selection based on the pH of your soil and the type of plants you are growing.
Phosphorus is held tightly to clay particles in the soil. If the soil test calls for more P, it is applied in the form of the chemical compound called phosphate, P2O5. Rock phosphate (30 to 36% P2O5) is a natural fertilizer that releases the phosphorus very slowly. When the pH is above 6.0, it becomes very water insoluble and not available to the plants. Concentrated triple superphosphate (46% P2O5) and regular superphosphate (20% P2O5) are the most commonly used single source of phosphorus.
Potassium may be lacking occasionally in soils. It is held tightly by the soil but not as much as phosphorus. It is applied in the form of the chemical compound called potash, K2O. Muriate of potash (60% K2O) is the most common source for the home gardener.
Magnesium and sulfur may be derived from common Epsom salts (10% Mg and 14% S). Magnesium is part of the chlorophyll molecule and a deficiency in the soil may lead to yellowing of leaves.
These elements are all part of the group commonly called macronutrients. Plants must have them in relatively large quantities in order to grow properly. Another group of elements called micronutrient or trace elements, are also required for plant growth but in much smaller amounts.
Iron (Fe), zinc (Zn) and copper (Cu) are micronutrients that are commonly applied as a chelate. A chelate is a synthetic organic substance that slowly releases the micronutrient and thus makes it available to the plant for a longer period. When these elements are applied in the normal inorganic salts form, they are not as available to the plant roots. Manganese (Mn) is the one exception where it is better applied as a salt rather than a chelate.
As represented by the high percentage numbers on these fertilizers, each product has a high level of salts present. Therefore, it takes only small amounts to accomplish the task. For instance, a standard application of nitrogen is usually 1 pound of N per 1,000 square feet of area. If you are using Urea at 45% nitrogen, you would only have to apply 2.2 pounds to 1,000 square feet. That is a relatively small amount to spread evenly over a fairly large area. If too much is applied close to the plant, it will “burn.” So, take care when applying these concentrated fertilizers onto or near growing plants.
